Abstract
Background:
Tyrosine kinase inhibitor (TKI) therapy is associated with the development of various pulmonary complications including pleural effusions (PE) and pulmonary arterial hypertension (PAH) when used in the treatment of chronic myeloid leukemia (CML). In this study, we aimed to evaluate the real-world incidence of pulmonary toxicities associated with TKIs, trends in management, discontinuations, subsequent therapies and overall outcomes.
Patients and Methods:
In this single-institution retrospective study, conducted at Abington Memorial Hospital, we reviewed charts of patients with CML treated with at least one TKI (imatinib, dasatinib, nilotinib, bosutinib and ponatinib). Patients with both front-line and subsequent-line therapy were included. Besides general demographics and disease specific characteristics, we examined dosing, pulmonary complications with their incidences, discontinuation rates, response rates and survival outcomes. χ2 test was used to determine associations and Kaplan-Meier test for time to event analysis.
Results:
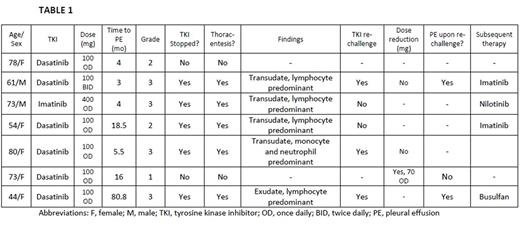
A total of 52 patients were included in this analysis. Median age at diagnosis was 68 years (range, 20-87), with 63.5% females, 78.8% Caucasians and 38.5% with smoking history. Baseline median white cell count was 53.2 x 103/µL (range, 8.4-505) and platelet count was 270 x 103/µL (range, 48-1740). At the initiation of TKI, 6 (11.5%) patients had cardiovascular and 1 (1.9%) had pulmonary disease. Forty-five patients received TKI as first-line therapy. Twenty-two (42.3%) patients received imatinib, 22 (42.3%) dasatinib, 19 (36.5%) nilotinib, 3 (5.7%) bosutinib and 2 (3.8%) ponatinib. Median duration of TKI therapy was 28.5 months. Overall incidence of PE was 13.5% (n = 7), PAH was 1.9% (n = 1) and lung parenchymal changes was 3.8% (n = 2). Six (27.2%) patients on dasatinib treatment developed PE (Table 1), 1 (4.5%) developed PAH and 2 (9%) developed lung parenchymal changes. All patients on dasatinib therapy who developed PE achieved major molecular remission (5 within 6 months and 1 within 18 months). Upon dasatinib re-initiation, 2 (67%) patients developed pleural effusion again. Age, smoking status, cardiovascular disease, pulmonary disease, baseline WBC count and Sokal score were not associated with increased risk of PE (p value > 0.1). Patient who did not develop PE had a higher mean estimated overall survival (174.8 [95% CI, 163.2-186.4] months]) as compared to those who developed PE (81.6 [95% CI, 59.1-104.1] months), however statistically significant difference was not found (p value = 0.18), Figure 1.
Conclusion:
The highest incidence of PE, PAH and lung parenchymal changes was found with dasatinib treatment, which is compatible with previously reported incidence. Management guidelines of these toxicities are unclear because many patients re-develop pleural effusions upon re-challenge with dasatinib, as demonstrated by our study. Hence, further studies are needed to determine optimal management strategy in these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.